Exercise is a powerful tool for enhancing mental performance and cognitive health. Whether you want to improve your memory[1][2], enhance your problem solving abilities[3], reduce your risk of Parkinson’s or Alzheimer’s[4][5][6][7], or bend metal and shoot lasers with your mind – exercise has an important role to play in all but two of those activities. In particular, richer exercise regimens – regimens consisting of aerobic and resistance training methods[8][1], produce the greatest overall improvements in cognitive performance. Note, however, working out for sixteen hours each day won’t turn you into the next Stephen Hawking. There are diminishing returns[9], and the favorable cerebral environment promoted by exercise is only as good as the active learning that accompanies it.
A quick word before diving deeper: for most fitness enthusiasts, bodybuilders, athletes etc. it is enough to know that certain actions can, with some degree of reliability, produce certain reactions. E.g. I want to improve my cardiovascular health, so I go swimming and my cardiovascular health improves. Or, I want to put on muscle mass, (simplifying out the nutrition and lifestyle aspects) so I pick up and put down heavy things repeatedly. We have confidence that these activities will produce results without referencing the underlying cellular and chemical processes. That said, compared to our understanding of exercise’s impact on cardiovascular health and general fitness, our understanding of exercise’s impact on cognitive health is still very much in its infancy. Despite very compelling research up to this point, the cause and effect relationships which seem so obvious in other areas still seem almost farcical in relation to the mind (bro, I’m gonna go exercise and get hella smart bro). In order to establish and popularize a tighter framework then, and not only because these systems are wildly interesting (caveat: the author’s opinion may or may not be a representative sample), we’ll cover these systems in some technical (but by no means extensive) detail. With that in mind, if the text starts to read like hieroglyphics, feel free to scan ahead to the summary figures or play around with the suggested workouts.
A Quick Intro to Neuroscience
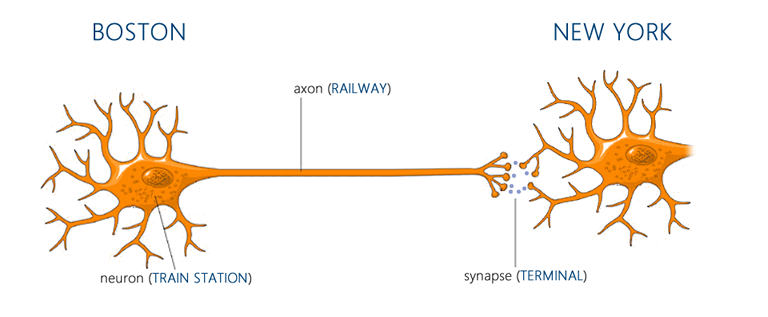
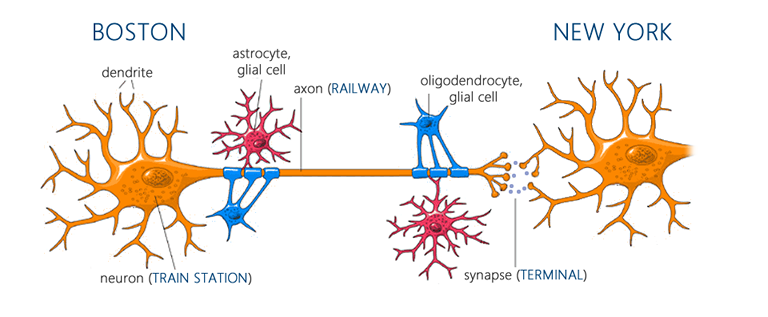
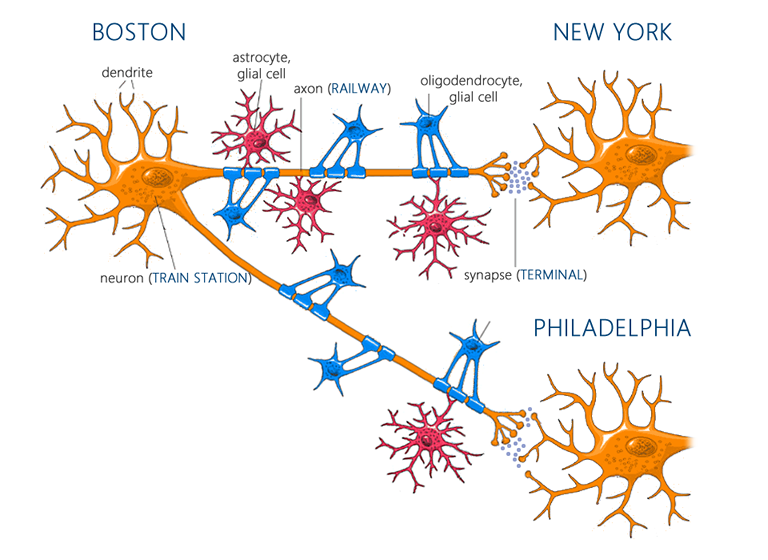
To understand how exercise exerts its positive effects on the brain, it’s helpful to have a basic framework in neuroscience. The primary functional units of the brain are neurons, which communicate with each other through projections called axons at junctions known as synapses[129]. When a neuron reaches a certain threshold of excitation, it triggers an ‘action potential’ which travels down its axon to its synapse with another neuron. At each synapse, chemicals called neurotransmitters are released, and, depending on the transmitter and the receptors on the post-synaptic neuron, these produce either an excitatory or inhibitory response. Note that your brain is composed of many billions of neurons, each with many thousands of synapses, communicating through a plethora of neurotransmitters and receptor subtypes. Γεια σας αγαπώ. If that was Greek to you, permit an analogy: each neuron can be viewed as a train station-like hub. Each neuronal ‘train station’ projects onto many other stations through its axons or ‘railways,’ and at the end of each rail-line there is a corresponding synapse or ‘terminal’ with another station. For this analogy, just imagine that each track of rail is one-way (there can be a connection from A to B and B to A, but when this occurs they are separate tracks), and that the same tracks always serve the same terminals. Whenever a station receives enough inbound trains at the right terminals, it sends off its own trains (action potentials) to the stations down the line[130]. Your brain then can be thought of as simply billions of these (Mega) Grand Central Stations, each connecting to thousands of other stations. Cool.

The Little Engine That Could as an Action Potential. So Meta.
Awesome, so now let’s say you want to learn Greek - how will that work, and how can exercise help? To answer that, it’s useful to disaggregate cognitive performance into its essential components. These are: laser-shooting, metal-bending, memory, problem solving, processing speed and attention [10]. While there are additional components of cognitive performance, much of the variance can be described by these four aspects – including much of what we might describe as learning ability or intelligence. While there is overlap between the regions, cells and neurotransmitters of the brain which underpin these processes, each process has its own unique profile. We will examine these in turn, with a primary focus on memory, and the potential impact exercise can have on them.
Memory
We’ll cover memory first, and in the greatest detail, as it is among the most extensively and intensively researched of our cognitive processes. Let's ignore for now the distinctions between types of memory, as well as methods of encoding, storage and retrieval. It is enough to know that many of the central functions of memory depend on the region of the brain known as the hippocampus (Latin for ‘seahorse’ referring to the shape of the brain region – I mention this only to prepare you for the terrible puns coming later)[11], and on processes of plasticity – the brain’s ability to dynamically alter neurons and the connections between them[12]. Without a functioning hippocampus, the process of memory encoding ceases to work, typically producing a state of amnesia[13](primarily anterograde amnesia, not retrograde amnesia – think 50 First Dates, not Anastasia). At the same time, improving the functioning of the hippocampus seems to be a reliable method of improving memory[14]. To see how exercise can impact memory and the hippocampus, it’s useful to distinguish between two broad types of benefits: general cellular and environmental impacts, and specific plasticity impacts.
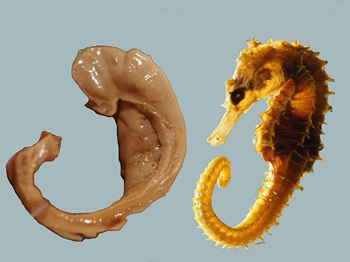
This is a hippocampus, not an alien embryo
In the same way that exercise has been shown to improve a wide range of general health conditions[15][16][17][18][19][20][21], so too does it have wide and fundamental health-promoting impacts across the brain. Its effects extend across vascular[22][23], metabolic[24][25], inflammatory[26] [24] and stress-regulating[27] pathways. Exercise has been shown to enhance neuronal metabolic and mitochondrial functioning[28][25], decrease oxidative stress in the hippocampus[29][27], and increase the levels of mitochondrial machinery [28]. Another way of saying this: exercise helps improve the power supply, the work environment and workers themselves in neuronal ‘train stations.’ On the other end, stress, and chronic stress in particular, has been linked to impaired functioning of, and indeed damage to, the hippocampus[27] (think of long term stress as a process that damages the structures of the train stations, like erosion or the creatures from Tremors). Exercise has shown favorable benefits in the regulation of stress (and in particular the management of corticosteroid processes – e.g. cortisol)[27], and may also favorably affect hippocampal memory systems through this process. Indirectly, by managing stress, exercise may also help enhance memory through a brain region known as the amygdala. The amygdala is a region of the brain heavily involved in emotion (for instance the amygdala would be partially responsible for the incredible feelings of joy you’re experiencing as you read this). Increased activation in the amygdala has been shown to modulate the hippocampus and enhance the process of memory formation[30]. Essentially, more emotionally salient events or information are stored more efficiently. For instance, if you were to start reading about the impending laser-cat apocalypse of 2013 (assuming, of course, you have a deep seated fear of cats, lasers, and a post-apocalyptic future) you might remember that more efficiently than the less emotionally salient information you read 10 lines ago. More practically, to the extent you care more about the information you’re attempting to learn, you are more likely to encode and successfully store that information. Regulating stress will help promote the healthy and beneficial functions of the amygdala[30], and the hippocampus in turn. In the short term, you may even attempt memorization or learning activities after a bout of exercise, as the signals which modulate the amygdala tend to be elevated after a good workout[31].

Laser Catopocalypse of 2013 – The Terror

As you can see here exercise helps fight stress. By shooting it. Right in the face.
More specific to the process of memory formation and the underlying neuronal structure, exercise has been shown to increase levels of hippocampal neurotrophins[32]. Neurotrophins are a class of growth factors centrally important in the development and health of neurons[33]. In particular, increases in a neurotrophin called brain derived neurotrophic factor (BDNF) have been linked to neurogenesis (the creation of new neurons) and improved synaptic plasticity[32][24] [34], producing substantial improvements in memory[24]. In turn, exercise has been shown to up-regulate levels of hippocampal BDNF[34]- and much of its favorable impact on memory appears to stem from this process[35]. To make a wild analogy and a gross, but potentially useful, simplification – BDNF is to neurogenesis as testosterone is to muscle growth. That is to say, quite helpful. (To extend the analogy, it’s worth noting that maximal neuronal growth would not translate to maximal intelligence just in the same way that maximum muscle cross-sectional area would not translate directly into maximum strength.) Similar to BDNF, insulin like growth factor 1 (IGF1) is another compound which has been shown to enhance neurogenesis and plasticity processes[36][37], and it is similarly upregulated through exercise[38][39][40]. Not only does exercise lead to favorable increases in these compounds, but it can also help regulate hormones which indirectly support them. That is, exercise has been shown to help maintain levels of testosterone and estrogen[41][42], which have also been shown to help favorably regulate levels of neurotrophins and neurogenesis [43][44][45][41]. Taken together, the neuronal and synaptic plasticity promoted by these compounds ultimately work to facilitate some of the basic processes of memory and learning. One of the critical mechanisms of memory formation and learning is a process called Long Term Potentiation, or ‘LTP.’ In LTP connections between neurons are enhanced by their firing in relation to one another[131]. That is, if neuron A connects to (or ‘synapses’ on) Neuron B, and neuron A fires right before Neuron B – the strength of the connection between those two neurons tends to be enhanced. Or rather, if a train from the Boston station always arrives in the New York station right before New York departures, the terminal between Boston and New York will be remodeled and enhanced. LTP is particularly important in the functioning of the hippocampus, and it appears that exercise has favorable effects on LTP [46][47][48], most likely mediated by its positive effects on synaptic plasticity generally (or ‘terminal remodeling and expansion’).
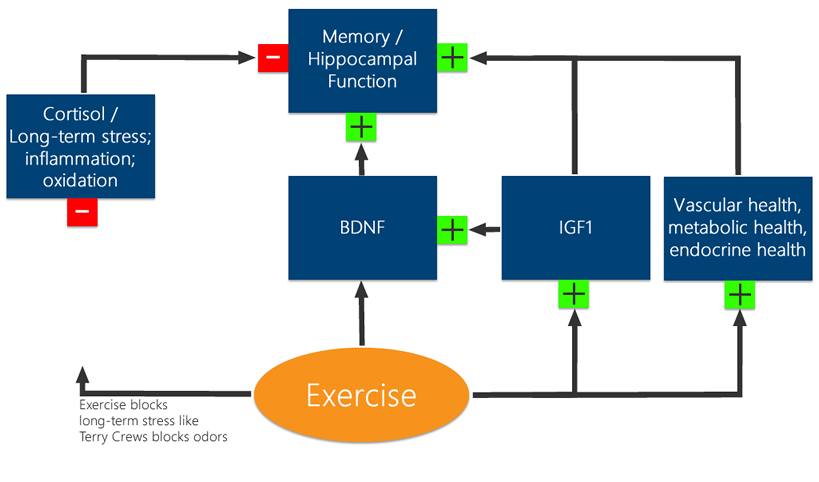

So Cortisol is all like "Memory, I don't like your face" - but Exercise is all like "Cortisol, come at me bro!" and Exercise, I mean this guy is just unbrolievable - he's like "Yo IGF1, BDNF - Memory is a broheim - we gotta have his back!" And they're all like "BROTALLY!" And that's how Exercise, IGF1 and BNDF create a membroble event. Scientifically.
The impacts of exercise on brain plasticity extend beyond neurons and their connections to also favorably regulate the brain’s supporting glial cells (pronounced ‘Glee-uhl ‘- but with no known musicals). Exercise has been shown to upregulate two types of glial cells worth particular consideration: astrocytes[49][22] and oligodendrocytes[50][51]. Astrocytes help give structure to the brain, provide vascular support and play a critical role in the management and flow of cerebral spinal fluid[52][53]. These are foundational activities for the brain, and undoubtedly necessary for managing neurogenesis and other cerebral changes[54]. To extend the railway analogy, astrocytes help provide much of the infrastructure and transport for the train stations themselves. Oligodendrocytes, on the other hand, are a glial cell type critically important in the regulation of neuronal myelin[55] – that is, for the sheath that covers the axons, the neuronal projections. As enhanced myelination has been shown to improve transmission[56][57][58], exercise’s ability to enhance the production of oligodendrocytes[50] is significant. Extending the analogy again, these cells and the myelin they produce help to regulate the efficiency and quality of the actual railway lines - producing fast and shiny tracks.

Exercise can help upregulate neurogenesis, vascular supply, synaptic plasticity, neurotransmitter populations, myelination and axonal transmitition. BOOM.
Ok, exercise is good for the memory, but surely there’s more to learning and intelligence than that? Yes, learning is also caused by psychic energy, or ‘libido’ – and the dynamic tension between the ‘pleasure principle’ and the ‘reality principle’ (kidding, totally kidding, you thought I was going all Freudian on you didn’t you?). More seriously, there are indications that exercise can improve processing speed [59][9][60], problem solving [2][61] and attention processes[62]. Many of the same mechanisms of neurotrophins, neurogenesis, synaptic plasticity, neurovascular and metabolic processes are likely involved in these improvements. Behold! A flow chart on the unstoppable, superhuman power of exercise:

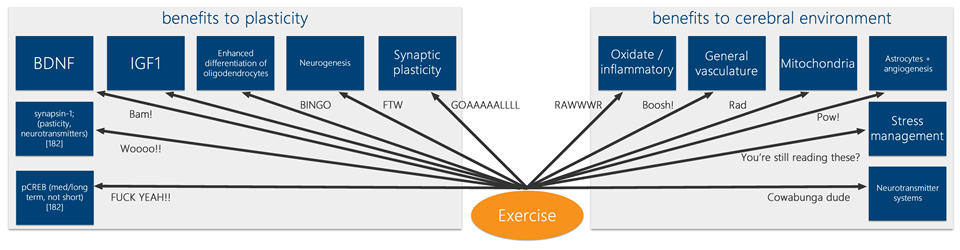
The Effects of Exercise on the Brain are Powerful and Diverse
Types of Exercise
Thus far, the methods of exercise have been described generally. Interestingly, most forms of exercise have been shown to benefit cognition. I.e. resistance exercise[63][64], aerobic exercise[65][9], and indeed simple flexibility and coordination exercises[66][62][67] have all been shown to produce some improvement on cognitive function. Of course, not all exercise is equivalent, and resistance and aerobic exercise have tended to show the greatest impacts on the brain[68][69][65][70]. Critically, however, while resistance and aerobic exercise have been shown to produce comparable effects, they appear to work through somewhat divergent pathways[1][71]. Aerobic exercise appears to yield a greater effect on vascular processes (no surprises there)[24][72] and BDNF[1], while resistance exercise appears to produce larger impacts on IGF1 pathways[1] and supporting hormones like estrogen in women and testosterone in men[73][74][75][76][77][78]. In then an awesome if perhaps unsurprising result, the greatest improvements in cognition tend to be observed with mixed-type exercise regimens[8]. That is, the combination of aerobic exercise with resistance exercise produces a greater overall improvement than either one individually. While clinical studies on extremely rich exercise regimens are limited, there is reason to believe that flexibility and coordination exercise on top of aerobic and resistance training could produce additional benefits [62][67]. Interestingly, these results mirror a general pattern seen in animal models of neurological development where richer environments lead to better overall neuronal and cerebral development[79]. It appears here that richer forms of physical stimulation (not that kind of ‘stimulation’) lead to similarly better outcomes in the brain.

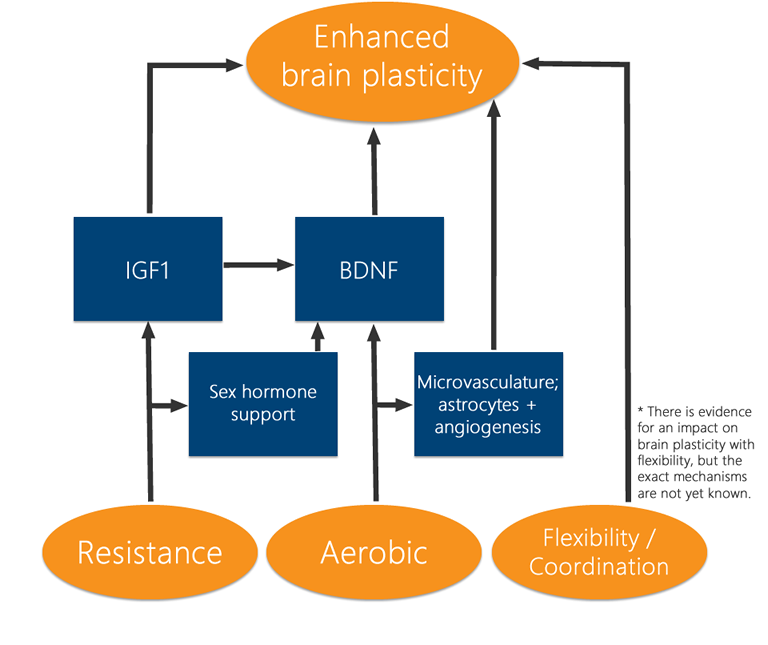

Types of Exercise: Better Together
Almost as interesting, the act of exercising itself appears to correlate more strongly with cognitive improvements than accumulated fitness capacity [80][66]. That is, at least in the short and medium term, exercise appears to be the necessary signal for upregulating neurotrophins and other signals for brain plasticity processes. This should both reassure the already fit crowd to maintain exercise habits, but also encourage the less fit to start exercising or to exercise more. Indeed, irrespective of couch or 5k status – exercise can begin to exert benefits on the brain.
Ok, ok, we get it. Exercise, and comprehensive exercise in particular, can have beneficial effects on the mind. Are you telling me that I should go out and run four hours every day, then pump Iron for six? No. As with many physical systems, the dose can make the medicine or the poison. There are indications that, at least on some cognitive measures, increasing exercise leads to diminishing returns and, beyond an extreme threshold, potentially impairment[81][9][82]. One classic model in cognitive psychology is the Yerkes-Dodson law – which claims a simple empirical relationship between arousal (no, not that kind of arousal – go read ‘Sex Olympian’ if that’s your bag) and cognitive performance. At low levels of arousal performance is diminished, at moderate levels performance is enhanced, and then again at the highest levels of arousal performance is once again relatively decreased. Interestingly, for simple tasks the optimal degree of arousal tends to be much higher than for complex tasks. The acute effect of exercise on cognition seems to follow a similar pattern [9]. To speak anecdotally for a moment, this author used to have a hell of a hard time programming, writing, or - for that matter - forming coherent audible sentences while doing two and three-a-days in college. The median thought looking something like: ‘f-f-foooood… sl-sle—sleeeeepy... foooooooood.’ That said, in the long term, while there will certainly be diminishing returns with more exercise, there is not enough clinical evidence to say whether cognitive performance will actually begin to cause impairment. If you love to workout, by all means keep it up.
Just because a little or moderate amount is good, doesn't mean a metric ton is better. "If I'm charming after 2 beers, imagine what a personality magnet I'll be after 20!!!" I can attest, it does not work like that.
Of course, it should go without saying that after ensuring the health of, even after optimizing, your underlying cognitive structures – you still have to go out and think [10]. In the same way a football player benefits from strength training, for all his effort in the weightroom he must still go play the game. Whether you’re creating artistic compositions, writing code, solving physics problems or crafting that perfectly witty text message your friends all know and love you for (that and your good looks and generosity, obviously) – there’s no reason to shortchange yourself by treating your body and mind poorly. Another way of saying this: you are responsible for writing and maintaining all of your own software. Whatever skills you need for your profession or passions, you will have to develop the neuronal connections and firing patterns on your own, and you will have to put in the time and work to do that. But, for all that, you must still respect the hardware and the operating system. The way you exercise, and your consistency with it could be the difference between bluescreening on Vista and, well, really almost anything else.

Beluga Screaming > Blue Screening
Cognitive Health
Ok, so now you’ve got your exercise on and you’re thinking clearly – what about the risks of disease and injury? Exercise. What? Exericse. No, but seriously, what do I do? Exercise. Ok, truthfully exercise is only going to reduce the risk, and is unlikely to be sufficient on its own. That said, there is compelling evidence that exercise can reduce the risk of Alzheimer’s, Parkinson’s and depression, and assist in recovery from traumatic brain injury (TBI for short – e.g. concussions). If my writing sounds like I’m suffering from a TBI of my own, stay with me here – we’ll cover some of the known physical processes underlying each disease/injury, and then provide some indications on how exercise can positively impact each process.
Let’s start with Alzheimer’s - a neurodegenerative disease characterized by the formation of plaques and fibrils in the brain. At least two primary events have been implicated in Alzheimer’s. The first is the formation and clumping of Beta-amyloid(Aβ), a protein that is formed by cleaving amyloid precursor protein (APP)[83]. APP by itself is harmless, and, indeed is important for proper neuronal functioning. However, in Alzheimer’s, once beta-amyloid has been cleaved from APP, groups of beta-amyloid proteins will tend to clump together, and these clumps may then interfere with proper functioning of nearby neurons[84]. The second mechanism behind Alzheimer’s is characterized as ‘taupathy’ – or dysfunction of the tau protein – a fundamental protein of the neuronal cytoskeleton. Dysfunction of the tau protein is very bad indeed for neuronal structure and transport, and can lead to neurofibrillary tangles[85][84](to return to the train station analogy, think of literally tangled and intertwined railway tracks; not good). While the exact chain of causation is unclear, it appears that beta amyloid plaques and clumps, and the neurofibrillary tangles lead to (or at a bare minimum are strongly correlated with) neurodegenerative conditions, which will tend to progressively worsen.
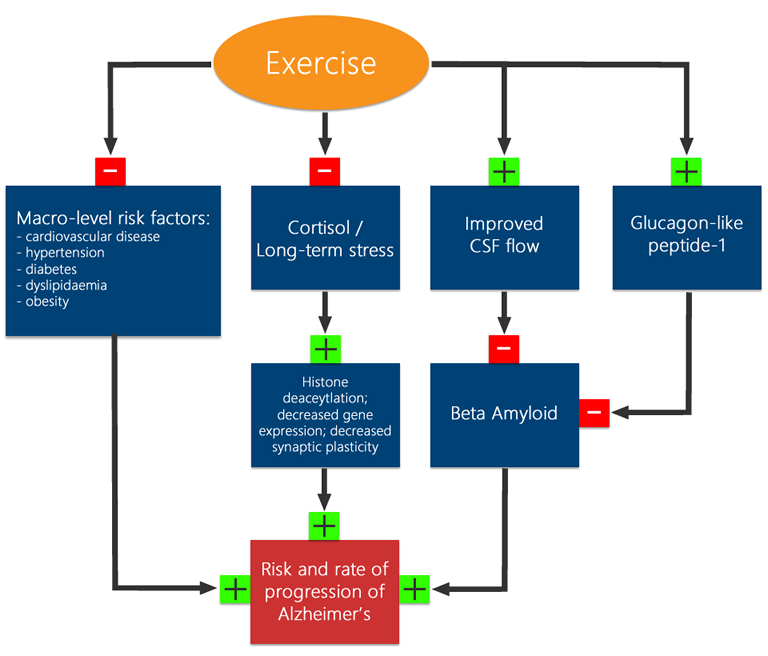
That said, the precise mechanism by which these pathologies develop and worsen are not fully understood. Beta-amyloid itself appears to have non-pathogenic functions[86], and it’s not clear whether the excessive aggregation of beta-amyloid is caused by increased production, lack of clearance by cerebrospinal fluid (CSF), or other mechanisms. While the root cause of Alzheimer’s is not yet well enough understood to propose a cure, methods of slowing its onset and mitigating its effects center around 1) preventing the formation of, or removing the Aβ and tau clumps from the brain and 2) preventing or mitigating the actual damage the clumps cause. In the first case, the removal of Aβ and tau errors, exercise appears to have a role to play. In an Alzheimer’s model, exercise has been shown to increase the health of astrocytes [5], a type of glial cell shown to be critically important in the flow of CSF[53]. In turn, CSF has been shown to play a key role in ‘cleaning’ neuronal structures, and scavenging Aβ in particular [53]. Indeed studies have shown high intensity exercise [87] is associated with lower levels of both tau and Aβ proteins in CSF [87]. In addition to CSF functioning, glucagon-like-peptide-1 (GLP1)– a metabolic peptide produced in the intestines, has also been shown to help clear Aβ [88][89], and is similarly favorably regulated by exercise[90]. Even once clumps and tangles have formed, exercise appears to mitigate the effects of Alzheimer’s. GLP1 itself exerts neuroprotective effects [88], and exercise has also been shown to help prevent histone de-acetylation, thereby maintaining more gene expression, thereby maintaining better neuronal and synaptic plasticity and health[91]. As well, the hippocampus is disproportionately affected in early Alzheimer’s [4], and therefore many of the systems discussed in memory will also act to mitigate some of the effects of Alzheimer’s. In terms of maintaining general cerebral health, it’s worth noting that many epidemiological risks for Alzheimer’s disease – such as cardiovascular disease, diabetes, obesity and hypertension – are quite malleable and have been shown to be positively influenced by exercise[23][15]. Indeed, in patients with early onset Alzheimer’s, cardiovascular fitness was positively correlated with larger parietal and temporal lobe volume [4]. Finally, the clincher, exercise has been shown to favorably impact the outcomes in AZ patients directly [92][87][93][94].

Parkinsons’ disease is another debilitating disorder of the central nervous system. Parkinson’s disease is associated with a loss of dopamine (a key neurotransmitter) producing cells in a region of the brain called the substantia nigra[95]. One of the central effects of Parkinson’s, also being one of the most debilitating, is bradykinesia, or slowness of movement[96]. Resistance exercise has been shown to combat some of the effects of Parkinson’s, and bradykinesia in particular. Indeed bradykinesia is a risk factor in and of itself (e.g. for falls and injuries), and many of the fundamental neural mechanisms that are involved in and improved by progressive strength training, like motor unit recruitment, synchronization, rate coding etc. may be useful in maintaining movement patterns [97]. In studies of rodent models of Parkinson’s, exercise has been shown to help improve impaired motor function, improve migration of cells to the damaged brain region, and otherwise enhance neuroprotection and/or neuronal differentiation[98]. Exercise has also been shown to mitigate the overall depletion of dopamine, as well as attenuate the loss of domapinergic neurons [99][100]. Critically, in actual human studies of Parkinson’s patients, exercise has been shown to ameliorate many of the physical declines typically seen in Parkinson’s [101][6][102][103].
It’s worth discussing the actual causes of Parkinson’s, and whether or not exercise may impact them. Of course, the current state of the research is far from conclusive, but it appears that Parkinson’s disease is at least partly associated with a protein called alpha-synuclein (a-syn). This protein has been shown to aggregate – in a process involving calcium channels and mis-regulated dopamine[104] — in Parkinson’s Disease. In turn, it appears that these aggregations displace and interfere with normal cell bodies [105][106][107][108][109][110][111][112]. This should sound somewhat familiar to the disorder seen in Alzheimer’s – where aggregations of beta amyloid may start to interfere with the health of the neural network. To take things further, but to enter the realm of speculation: just as cerebrospinal fluid appears to play a role in cleaning beta amyloid, so too might it serve that purpose with alpha-synuclein. Diminished levels of alpha-synuclein have been seen in the CSF of Parkinson’s patients [110][113], and it’s quite possible that this is at least partially a function of the diminished functioning of the CSF ‘glymphatic’ system (analogous to the lymph system in the rest of the body – here promoted by ‘glial’ cells – hence the ‘glymphatic’ nickname). I.e. either diminished CSF volume, or more likely diminished CSF rate or efficiency of flow could result in deficiency of the CSF’s ‘cleaning’ mechanism. This in turn could lead or contribute to the observed protein aggregations and impairments. To the extent that exercise can favorably impact the CSF system (again, correlative, not definitive data) – and the general neurovascular and metabolic systems that are likely to form its bedrock – exercise may have an important role to play in delaying the onset and rate of progression of Parkinson’s.
A word on traumatic brain injury (TBI): with a growing awareness of concussions (the most common form of TBI) and the risks they pose both to the long term health of the brain and nervous system, a review of exercise’s impact on cognition would not be complete without some treatment of TBIs. TBIs are no trifling matter, they can impair cognitive performance[114], and the effects can last for years [115]. While the location and effect of TBIs can be extraordinarily diverse, certain patterns of impairment and recovery have been demonstrated. Exercise’s impact on these processes is contingent on the stage of recovery. Too soon after the injury, in the initial, roughly 2 week period, immediately following a TBI and exercise has been shown to negatively impact outcomes [116]. However, once the early window of recovery is through, exercise has been shown to have a range of positive effects on the recovery process. These include improving the brain’s natural adaptive processes in response to injury [117], reducing cerebral inflammation [26], and general neuroprotective and plasticity effects[116]. Similarly, concussions have been shown to disrupt hippocampal function[118], and, in case I haven’t beaten the dead seahorse (did you see what I did there?) enough, exercise has been shown to have favorable effects on the hippocampus.

Wile E Coyote guide to TBIs. Step 1: Rest; Step 2: Continue "I Will Get That Road Runner if it's the Last Thing I Ever Do" Exercise Regimen
[As a disclaimer, and this should go without saying, but every type of TBI is different and should be handled contextually with appropriate medical supervision. E.g. a mild bump vs. a pipe through the head warrant different treatment strategies, and doctors should be used to identify them.]
Depression is a complex phenomenon, and, I don’t have to say it – no fun at all. As well, depression itself can have deleterious effects on other aspects of cognitive health and performance[119]. Enter, once more, our hero: exercise. Exercise has been shown to favorably impact key neurotransmitter systems implicated in depression, including the dopaminergic, noradrenergic and serotonergic systems [27][61]. Exercise has also been shown to help regulate glucocorticoids and stress, which have themselves been correlated with depression [119][27]. Moreover, our plasticity promoting heroes IGF1 and BDNF have also been linked to favorable outcomes in depression and have been hypothesized as treatments [37]. Critically of course, in real world human studies exercise has also been shown to improve and help alleviate depression – especially in the elderly [24][120][121].
Finally, because its impacts on Alzheimer’s, Parkinson’s, TBIs and depression clearly aren’t enough, exercise has also been shown – again and again – to ameliorate general cognitive decline[122][123][69][124][125]. Ok, the point has been made – now a qualifier: exercise is unlikely, by itself, to cure your disease or keep you alive and sharp until 150. That said, there are overwhelming indications that it is a beneficial tool for managing the risk and rate of progression of all of the conditions mentioned, and general aging in particular. Of course, if exercise’s effects on the cardiovascular system[19][20], metabolic system[21], inflammatory processes[24][16], and mind-laser-shooting [not really] had no additional impact on cognitive processes (even though those macro-level processes DO impact the healthful functioning of the brain), it would still be worth doing. The value of exercise needs no further belaboring.

It’s clear that the brain is highly complex (yes, hold the applause for that deep insight), and its health and function, particularly in the long term, depend upon many tightly intertwined systems. It should come as no surprise then, that the methods of exercise to maintain and promote cognitive performance and health are themselves also diverse; their benefits extending across a myriad of cellular and chemical processes in the brain. From specific plasticity processes to general metabolic functioning, exercise helps to create a favorable environment for learning as well as a robust environment to help fight neurodegeneration. Whether starting from a high degree of fitness or a low one, exercise in and of itself – including resistance, aerobic and flexibility/coordination activities, has been shown to favorably impact the neurochemical environment. While exercising will not magically transpose a knowledge of quantum physics onto your cortex, it has been shown time and again to create a favorable neural environment, and one that will facilitate the learning you choose to work at. While we’re not all after maximal mental performance, quality and length of life are nearly universal concerns. Here, again, our friend exercise shines – reducing the risks of mental diseases, mitigating and reducing their rates of onset and damage, and generally managing and slowing cognitive decline. It may not be a panacea, but a rich consistent exercise regimen can give you a little more quality time before riding your seahorse into the sunset.


Sources:
[1] Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R, de Mello MT. (2011) Spatial Memory Is Improved By Aerobic And Resistance Exercise Through Divergent Molecular Mechanisms https://www.ncbi.nlm.nih.gov/pubmed/22155655
[2] Berchtold NC, Castello N, Cotman CW. (2010) Exercise And Time-Dependent Benefits To Learning And Memory https://www.ncbi.nlm.nih.gov/pubmed/20219647
[3] McMorris T, Davranche K, Jones G, Hall B, Corbett J, Minter C. (2009) Acute Incremental Exercise, Performance Of A Central Executive Task, And Sympathoadrenal System And Hypothalamic-Pituitary-Adrenal Axis Activity. https://www.ncbi.nlm.nih.gov/pubmed/19454298
[4] Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. (2009) Cardiorespiratory Fitness And Preserved Medial Temporal Lobe https://www.ncbi.nlm.nih.gov/pubmed/19812458
[5] Rodrigues L, Dutra MF, Ilha J, Biasibetti R, Quincozes-Santos A, Leite MC, Marcuzzo S, Achaval M, Gonçalves CA. (2010) Treadmill Training Restores Spatial Cognitive Deficits And Neurochemical Alterations In The Hippocampus Of Rats Submitted To An Intracerebroventricular Administration Of Streptozotocin. https://www.ncbi.nlm.nih.gov/pubmed/20953641
[6] Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. (2008) The Effectiveness Of Exercise Interventions For People With Parkinson'S Disease: A Systematic Review And Meta-Analysis. https://www.ncbi.nlm.nih.gov/pubmed/18181210
[7] Yuen-Sum Lau, Gaurav Patki, Kaberi Das-Panja, Wei-Dong Le, and S. Omar Ahmad (2011) Neuroprotective Effects And Mechanisms Of Exercise In A Chronic Mouse Model Of Parkinson’S Disease With Moderate Neurodegeneration https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079264/
[8] Stanley Colcombe and Arthur F. Kramer (2003) Fitness Effects On The Cognitive Function Of Older Adults A Meta-Analytic Study https://pss.sagepub.com/content/14/2/125.abstract
[9] Tomporowski PD. (2003) Effects Of Acute Bouts Of Exercise On Cognition https://www.ncbi.nlm.nih.gov/pubmed/12595152
[10] Michelene T.H. Chi, Robert Glaser, Ernest Rees (1982) Expertise In Problem Solving https://www.public.asu.edu/~mtchi/papers/ChiGlaserRees.pdf
[11] Squire LR. (1992) Memory And The Hippocampus: A Synthesis From Findings With Rats, Monkeys, And Humans. https://www.ncbi.nlm.nih.gov/pubmed/1594723
[12] S. F. Cooke and T. V. P. Bliss (2006) Plasticity In The Human Central Nervous System https://brain.oxfordjournals.org/content/129/7/1659
[13] Cipolotti L, Bird CM. (2006) Amnesia And The Hippocampus https://www.ncbi.nlm.nih.gov/pubmed/17102699
[14] Mangina CA, Sokolov EN. (2006) Neuronal Plasticity In Memory And Learning Abilities: Theoretical Position And Selective Review. https://www.ncbi.nlm.nih.gov/pubmed/16387375
[15] Hu G, Lakka TA, Kilpeläinen TO, Tuomilehto J. (2007) Epidemiological Studies Of Exercise In Diabetes Prevention. https://www.ncbi.nlm.nih.gov/pubmed/17510700
[16] Woods JA, Vieira VJ, Keylock KT. (2006) Exercise, Inflammation, And Innate Immunity. https://www.ncbi.nlm.nih.gov/pubmed/16877125
[17] Topp R, Fahlman M, Boardley D. (2004) Healthy Aging: Health Promotion And Disease Prevention. https://www.ncbi.nlm.nih.gov/pubmed/15159189
[18] Antignani PL. (2003) Treatment Of Chronic Peripheral Arterial Disease. https://www.ncbi.nlm.nih.gov/pubmed/15320844
[19] Hu FB. (2009) Diet And Lifestyle Influences On Risk Of Coronary Heart Disease. https://www.ncbi.nlm.nih.gov/pubmed/19500488
[20] Böhm M, Werner C, Jakobsen A, Heroys J, Ralph A, Rees T, Shaw M. (2008) Treating To Protect: Current Cardiovascular Treatment Approaches And Remaining Needs. https://www.ncbi.nlm.nih.gov/pubmed/18449384
[21] Lakka TA, Laaksonen DE. (2007) Physical Activity In Prevention And Treatment Of The Metabolic Syndrome. https://www.ncbi.nlm.nih.gov/pubmed/17332786
[22] Li J, Ding YH, Rafols JA, Lai Q, McAllister JP 2nd, Ding Y. (2005) Increased Astrocyte Proliferation In Rats After Running Exercise https://www.ncbi.nlm.nih.gov/pubmed/16024173
[23] Kalaria RN. (2010) Vascular Basis For Brain Degeneration Faltering Controls And Risk Factors For Dementia https://www.ncbi.nlm.nih.gov/pubmed/21091952
[24] Cotman CW, Berchtold NC, Christie LA. (2007) Exercise Builds Brain Health: Key Roles Of Growth Factor Cascades And Inflammation https://www.ncbi.nlm.nih.gov/pubmed/17765329
[25] Gomez-Pinilla F, Vaynman S, Ying Z. (2008) Brain-Derived Neurotrophic Factor Functions As A Metabotrophin To Mediate The Effects Of Exercise On Cognition. https://www.ncbi.nlm.nih.gov/pubmed/19046371
[26] Archer T, Svensson K, Alricsson M. (2012) Physical Exercise Ameliorates Deficits Induced By Traumatic Brain Injury. https://www.ncbi.nlm.nih.gov/pubmed/22233115
[27] Tsatsoulis A, Fountoulakis S. (2006) The Protective Role Of Exercise On Stress System Dysregulation And Comorbidities https://www.ncbi.nlm.nih.gov/pubmed/17148741
[28] Marcelo O. Dietrich, Zane B. Andrews, and Tamas L. Horvath (2008) Exercise-Induced Synaptogenesis In The Hippocampus Is Dependent On Ucp2-Regulated Mitochondrial Adaptation https://www.jneurosci.org/content/28/42/10766.full
[29] Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. (2007) Effects Of Exercise On Brain Function: Role Of Free Radicals https://www.ncbi.nlm.nih.gov/pubmed/18059620
[30] Roozendaal B, McEwen BS, Chattarji S. (2009) Stress, Memory And The Amygdala. https://www.ncbi.nlm.nih.gov/pubmed/19469026
[31] Goto K, Ishii N, Kizuka T, Kraemer RR, Honda Y, Takamatsu K. (2009) Hormonal And Metabolic Responses To Slow Movement Resistance Exercise With Different Durations Of Concentric And Eccentric Actions. https://www.ncbi.nlm.nih.gov/pubmed/19430944
[32] Cotman CW, Berchtold NC. (2002) Exercise: A Behavioral Intervention To Enhance Brain Health And Plasticity https://www.ncbi.nlm.nih.gov/pubmed/12086747
[33] Louis F Reichardt (2006) Neurotrophin-Regulated Signalling Pathways https://rstb.royalsocietypublishing.org/content/361/1473/1545.short
[34] Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. (2012) Bdnf Promotes Differentiation And Maturation Of Adult-Born Neurons Through Gabaergic Transmission. https://www.ncbi.nlm.nih.gov/pubmed/23055503
[35] Yamada K, Nabeshima T. (2003) Brain-Derived Neurotrophic Factor/Trkb Signaling In Memory Processes. https://www.ncbi.nlm.nih.gov/pubmed/12719654
[36] Aberg ND, Brywe KG, Isgaard J. (2006) Aspects Of Growth Hormone And Insulin-Like Growth Factor-I Related To Neuroprotection, Regeneration, And Functional Plasticity In The Adult Brain https://www.ncbi.nlm.nih.gov/pubmed/16432628
[37] Paslakis G, Blum WF, Deuschle M. (2012) Intranasal Insulin-Like Growth Factor I (Igf-I) As A Plausible Future Treatment Of Depression. https://www.ncbi.nlm.nih.gov/pubmed/22626951
[38] Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. (2007) Exercise Intensity Influences The Temporal Profile Of Growth Factors Involved In Neuronal Plasticity Following Focal Ischemia. https://www.ncbi.nlm.nih.gov/pubmed/17382914
[39] Markowska AL, Mooney M, Sonntag WE. (1998) Insulin-Like Growth Factor-1 Ameliorates Age-Related Behavioral Deficits. https://www.ncbi.nlm.nih.gov/pubmed/9758223
[40] Borst SE, De Hoyos DV, Garzarella L, Vincent K, Pollock BH, Lowenthal DT, Pollock ML. (2001) Effects Of Resistance Training On Insulin-Like Growth Factor-I And Igf Binding Proteins https://www.ncbi.nlm.nih.gov/pubmed/11283443
[41] Okamoto M, Hojo Y, Inoue K, Matsui T, Kawato S, McEwen BS, Soya H. (2012) Mild Exercise Increases Dihydrotestosterone In Hippocampus Providing Evidence For Androgenic Mediation Of Neurogenesis. https://www.ncbi.nlm.nih.gov/pubmed/22807478
[42] Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. (2001) Estrogen And Exercise Interact To Regulate Brain-Derived Neurotrophic Factor Mrna And Protein Expression In The Hippocampus https://www.ncbi.nlm.nih.gov/pubmed/11860494
[43] Handa RJ, Ogawa S, Wang JM, Herbison AE. (2012) Roles For Oestrogen Receptor Β In Adult Brain Function. https://www.ncbi.nlm.nih.gov/pubmed/21851428
[44] Galea LA, Spritzer MD, Barker JM, Pawluski JL. (2006) Gonadal Hormone Modulation Of Hippocampal Neurogenesis In The Adult. https://www.ncbi.nlm.nih.gov/pubmed/16411182
[45] Galea LA. (2008) Gonadal Hormone Modulation Of Neurogenesis In The Dentate Gyrus Of Adult Male And Female Rodents. https://www.ncbi.nlm.nih.gov/pubmed/17669502
[46] van Praag H, Christie BR, Sejnowski TJ, Gage FH. (1999) Running Enhances Neurogenesis, Learning, And Long-Term Potentiation In Mice https://www.ncbi.nlm.nih.gov/pubmed/10557337
[47] Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. (2004) Effects Of Voluntary Exercise On Synaptic Plasticity And Gene Expression In The Dentate Gyrus Of Adult Male Sprague–Dawley Rats In Vivo https://www.ncbi.nlm.nih.gov/pubmed/14960340
[48] Nagappan G, Lu B. (2005) Activity-Dependent Modulation Of The Bdnf Receptor Trkb: Mechanisms And Implications https://www.ncbi.nlm.nih.gov/pubmed/16040136
[49] Uda M, Ishido M, Kami K, Masuhara M. (2006) Effects Of Chronic Treadmill Running On Neurogenesis In The Dentate Gyrus Of The Hippocampus Of Adult Rat https://www.ncbi.nlm.nih.gov/pubmed/16824490
[50] Simon C, Götz M, Dimou L. (2011) Progenitors In The Adult Cerebral Cortex: Cell Cycle Properties And Regulation By Physiological Stimuli And Injury. https://www.ncbi.nlm.nih.gov/pubmed/21446038
[51] Krityakiarana W, Espinosa-Jeffrey A, Ghiani CA, Zhao PM, Topaldjikian N, Gomez-Pinilla F, Yamaguchi M, Kotchabhakdi N, de Vellis J. (2010) Voluntary Exercise Increases Oligodendrogenesis In Spinal Cord. https://www.ncbi.nlm.nih.gov/pubmed/20374076
[52] Parri R, Crunelli V. (2003) An Astrocyte Bridge From Synapse To Blood Flow. https://www.ncbi.nlm.nih.gov/pubmed/12494240
[53] Jeffrey J. Iliff, Minghuan Wang, Yonghong Liao, Benjamin A. Plogg, Weiguo Peng, Georg A. Gundersen, Helene Benveniste, G. Edward Vates, Rashid Deane, Steven A. Goldman, Erlend A. Nagelhus and Maiken Nedergaard (2012) A Paravascular Pathway Facilitates Csf Flow Through The Brain Parenchyma And The Clearance Of Interstitial Solutes, Including Amyloid Β https://stm.sciencemag.org/content/4/147/147ra111
[54] Zaheer A, Haas JT, Reyes C, Mathur SN, Yang B, Lim R. (2006) Gmf-Knockout Mice Are Unable To Induce Brain-Derived Neurotrophic Factor After Exercise https://www.ncbi.nlm.nih.gov/pubmed/16758368
[55] Baumann N, Pham-Dinh D. (2001) Biology Of Oligodendrocyte And Myelin In The Mammalian Central Nervous System. https://www.ncbi.nlm.nih.gov/pubmed/11274346
[56] Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. (2005) Extensive Piano Practicing Has Regionally Specific Effects On White Matter Development. https://www.ncbi.nlm.nih.gov/pubmed/16116456
[57] Fields RD. (2005) Myelination: An Overlooked Mechanism Of Synaptic Plasticity? https://www.ncbi.nlm.nih.gov/pubmed/16282593
[58] R. Douglas Fields (2008) White Matter In Learning, Cognition And Psychiatric Disorders https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2486416/
[59] Fontana FE, Mazzardo O, Mokgothu C, Furtado O Jr, Gallagher JD. (2009) Influence Of Exercise Intensity On The Decision-Making Performance Of Experienced And Inexperienced Soccer Players. https://www.ncbi.nlm.nih.gov/pubmed/19454768
[60] Hogervorst E, Riedel W, Jeukendrup A, Jolles J. (1996) Cognitive Performance After Strenuous Physical Exercise. https://www.ncbi.nlm.nih.gov/pubmed/8902021
[61] Kramer AF, Erickson KI, Colcombe SJ. (2006) Exercise, Cognition, And The Aging Brain https://www.ncbi.nlm.nih.gov/pubmed/16778001
[62] Hötting K, Reich B, Holzschneider K, Kauschke K, Schmidt T, Reer R, Braumann KM, Röder B. (2012) Differential Cognitive Effects Of Cycling Versus Stretching/Coordination Training In Middle-Aged Adults https://www.ncbi.nlm.nih.gov/pubmed/21895371
[63] Suijo K, Inoue S, Ohya Y, Odagiri Y, Takamiya T, Ishibashi H, Itoh M, Fujieda Y, Shimomitsu T. (2012) Resistance Exercise Enhances Cognitive Function In Mouse. https://www.ncbi.nlm.nih.gov/pubmed/23041964
[64] Liu-Ambrose T, Donaldson MG. (2009) Exercise And Cognition In Older Adults: Is There A Role For Resistance Training Programmes? https://www.ncbi.nlm.nih.gov/pubmed/19019904
[65] Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. (1999) Ageing, Fitness And Neurocognitive Function. https://www.ncbi.nlm.nih.gov/pubmed/10440369
[66] Holzschneider K, Wolbers T, Röder B, Hötting K. (2012) Cardiovascular Fitness Modulates Brain Activation Associated With Spatial Learning https://www.ncbi.nlm.nih.gov/pubmed/22027496
[67] Reynolds D, Nicolson RI. (2007) Follow-Up Of An Exercise-Based Treatment For Children With Reading Difficulties. https://www.ncbi.nlm.nih.gov/pubmed/17557685
[68] Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. (2011) Exercise, Brain, And Cognition Across The Life Span https://www.ncbi.nlm.nih.gov/pubmed/21527670
[69] Lindsay S. Nagamatsu, MA, MA; Todd C. Handy, PhD, PhD; C. Liang Hsu, BSc, BSc; Michelle Voss, PhD, PhD; Teresa Liu-Ambrose, PT, PhD, PT, PhD (2012) Resistance Training Promotes Cognitive And Functional Brain Plasticity In Seniors With Probable Mild Cognitive Impairment https://archinte.jamanetwork.com/article.aspx?articleid=1135414
[70] Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L (2008) Physical Activity And Enhanced fitness To Improve Cognitive Function In Older People Without Known Impairment https://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005381.pub2/pdf
[71] Lin TW, Chen SJ, Huang TY, Chang CY, Chuang JI, Wu FS, Kuo YM, Jen CJ. (2012) Different Types Of Exercise https://www.ncbi.nlm.nih.gov/pubmed/22085720
[72] Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. (2007) The Impact Of Resistance Exercise On The Cognitive Function Of The Elderly. https://www.ncbi.nlm.nih.gov/pubmed/17762374
[73] Hackney AC. (2008) Effects Of Endurance Exercise On The Reproductive System Of Men: The "Exercise-Hypogonadal Male Condition". https://www.ncbi.nlm.nih.gov/pubmed/19092301
[74] Enea C, Boisseau N, Fargeas-Gluck MA, Diaz V, Dugué B. (2011) Circulating Androgens In Women: Exercise-Induced Changes. https://www.ncbi.nlm.nih.gov/pubmed/21142281
[75] Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. (2010) Testosterone Physiology In Resistance Exercise And Training: The Up-Stream Regulatory Elements. https://www.ncbi.nlm.nih.gov/pubmed/21058750
[76] Walker S, Taipale RS, Nyman K, Kraemer WJ, Häkkinen K. (2011) Neuromuscular And Hormonal Responses To Constant And Variable Resistance Loadings. https://www.ncbi.nlm.nih.gov/pubmed/20473217
[77] Kraemer WJ, Ratamess NA. (2005) Hormonal Responses And Adaptations To Resistance Exercise And Training. https://www.ncbi.nlm.nih.gov/pubmed/15831061
[78] Moghadasi M, Siavashpour S. (2012) The Effect Of 12 Weeks Of Resistance Training On Hormones Of Bone Formation In Young Sedentary Women. https://www.ncbi.nlm.nih.gov/pubmed/22562545
[79] Lewis MH. (2004) Environmental Complexity And Central Nervous System Development And Function. https://www.ncbi.nlm.nih.gov/pubmed/15362162
[80] Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S. (2010) Physical Activity And Memory Functions: Are Neurotrophins And Cerebral Gray Matter Volume The Missing Link? https://www.ncbi.nlm.nih.gov/pubmed/19853041
[81] Moore RD, Romine MW, O'connor PJ, Tomporowski PD. (2012) The Influence Of Exercise-Induced Fatigue On Cognitive Function. https://www.ncbi.nlm.nih.gov/pubmed/22494399
[82] Ploughman M. (2008) Exercise Is Brain Food: The Effects Of Physical Activity On Cognitive Function https://www.ncbi.nlm.nih.gov/pubmed/18781504
[83] Vassar R. (2002) Beta-Secretase (Bace) As A Drug Target For Alzheimer'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/12453676
[84] Ghiso J, Frangione B. (2002) Amyloidosis And Alzheimer'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/12453671
[85] Mudher A, Lovestone S. (2002) Alzheimer'S Disease-Do Tauists And Baptists Finally Shake Hands? https://www.ncbi.nlm.nih.gov/pubmed/11801334
[86] Debomoy K. Lahiri, Bryan Maloney (2010) Beyond The Signaling Effect Role Of Amyloid–Β42 On The Processing Of Aβpp, And Its Clinical Implications https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2922469/
[87] Baker LD, Bayer-Carter JL, Skinner J, Montine TJ, Cholerton BA, Callaghan M, Leverenz JB, Walter BK, Tsai E, Postupna N, Lampe J, Craft S. (2012) High-Intensity Physical Activity Modulates Diet Effects On Cerebrospinal Amyloid-Β Levels In Normal Aging And Mild Cognitive Impairment. https://www.ncbi.nlm.nih.gov/pubmed/21971406
[88] Bak AM, Egefjord L, Gejl M, Steffensen C, Stecher CW, Smidt K, Brock B, Rungby J. (2011) Targeting Amyloid-Beta By Glucagon-Like Peptide -1 (Glp-1) In Alzheimer'S Disease And Diabetes. https://www.ncbi.nlm.nih.gov/pubmed/21749267
[89] Li L. (2007) Is Glucagon-Like Peptide-1, An Agent Treating Diabetes, A New Hope For Alzheimer’S Disease? https://www.ncbi.nlm.nih.gov/pubmed/17592527
[90] Stensel D. (2010) Exercise, Appetite And Appetite-Regulating Hormones: Implications For Food Intake And Weight Control. https://www.ncbi.nlm.nih.gov/pubmed/21346335
[91] Elsner VR, Lovatel GA, Bertoldi K, Vanzella C, Santos FM, Spindler C, de Almeida EF, Nardin P, Siqueira IR. (2011) Effect Of Different Exercise Protocols On Histone Acetyltransferases And Histone Deacetylases Activities In Rat Hippocampus. https://www.ncbi.nlm.nih.gov/pubmed/21745541
[92] Coelho FG, Santos-Galduroz RF, Gobbi S, Stella F. (2009) Systematized Physical Activity And Cognitive Performance In Elderly With Alzheimer'S Dementia: A Systematic Review https://www.ncbi.nlm.nih.gov/pubmed/19578690
[93] Fang Yu. (2011) Guiding Research And Practice: A Conceptual Model For Aerobic Exercise Training In Alzheimer'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/21429955
[94] Archer T. (2011) Physical Exercise Alleviates Debilities Of Normal Aging And Alzheimer'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/20880302
[95] Smith Y, Villalba R. (2008) Striatal And Extrastriatal Dopamine In The Basal Ganglia: An Overview Of Its Anatomical Organization In Normal And Parkinsonian Brains. https://www.ncbi.nlm.nih.gov/pubmed/18781680
[96] J Jankovic (2008) Parkinson’S Disease: Clinical Features And Diagnosis https://jnnp.bmj.com/content/79/4/368.full
[97] David FJ, Rafferty MR, Robichaud JA, Prodoehl J, Kohrt WM, Vaillancourt DE, Corcos DM. (2012) Progressive Resistance Exercise And Parkinson’S Disease: A Review Of Potentialmechanisms https://www.ncbi.nlm.nih.gov/pubmed/22191068
[98] Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. (2010) Exercise Exerts Neuroprotective Effects On Parkinson'S Disease Model Of Rats https://www.ncbi.nlm.nih.gov/pubmed/19900418
[99] Poulton NP, Muir GD. (2005) Treadmill Training Ameliorates Dopamine Loss But Not Behavioral Deficits In Hemi-Parkinsonian Rats https://www.ncbi.nlm.nih.gov/pubmed/15817277
[100] Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. (2012) Neurorestoration By Physical Exercise: Moving Forward. https://www.ncbi.nlm.nih.gov/pubmed/22166417
[101] Tomlinson CL, Patel S, Meek C, Clarke CE, Stowe R, Shah L, Sackley CM, Deane KH, Herd CP, Wheatley K, Ives N. (2012) Physiotherapy Versus Placebo Or No Intervention In Parkinson'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/22786482
[102] Fernandez HH. (2012) Updates In The Medical Management Of Parkinson Disease. https://www.ncbi.nlm.nih.gov/pubmed/22219231
[103] Ahlskog JE. (2011) Does Vigorous Exercise Have A Neuroprotective Effect In Parkinson Disease? https://www.ncbi.nlm.nih.gov/pubmed/21768599
[104] Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. (2009) Interplay Between Cytosolic Dopamine, Calcium, And Alpha-Synuclein Causes Selective Death Of Substantia Nigra Neurons. https://www.ncbi.nlm.nih.gov/pubmed/19409267
[105] Tapia-González S, Giráldez-Pérez RM, Cuartero MI, Casarejos MJ, Mena MÁ, Wang XF, Sánchez-Capelo A. (2011) Dopamine And A-Synuclein Dysfunction In Smad3 Null Mice. https://www.ncbi.nlm.nih.gov/pubmed/21995845
[106] Michell AW, Tofaris GK, Gossage H, Tyers P, Spillantini MG, Barker RA. (2007) The Effect Of Truncated Human Alpha-Synuclein (1-120) On Dopaminergic Cells In A Transgenic Mouse Model Of Parkinson'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/17708336
[107] Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. (2000) Alpha-Synuclein Up-Regulation In Substantia Nigra Dopaminergic Neurons Following Administration Of The Parkinsonian Toxin Mptp. https://www.ncbi.nlm.nih.gov/pubmed/10646524
[108] St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. (2007) Dopaminergic Neuron Loss And Up-Regulation Of Chaperone Protein Mrna Induced By Targeted Over-Expression Of Alpha-Synuclein In Mouse Substantia Nigra. https://www.ncbi.nlm.nih.gov/pubmed/17241127
[109] Gründemann J, Schlaudraff F, Haeckel O, Liss B. (2008) Elevated Alpha-Synuclein Mrna Levels In Individual Uv-Laser-Microdissected Dopaminergic Substantia Nigra Neurons In Idiopathic Parkinson'S Disease. https://www.ncbi.nlm.nih.gov/pubmed/18332041
[110] Kasuga K, Ikeuchi T. (2012) Cerebrospinal Fluid And Plasma Biomarkers For Dementia With Lewy Bodies https://www.ncbi.nlm.nih.gov/pubmed/22570064
[111] Eller M, Williams DR. (2011) Α-Synuclein In Parkinson Disease And Other Neurodegenerative Disorders. https://www.ncbi.nlm.nih.gov/pubmed/21342025
[112] Eller M, Williams DR. (2009) Biological Fluid Biomarkers In Neurodegenerative Parkinsonism. https://www.ncbi.nlm.nih.gov/pubmed/19724250
[113] Mollenhauer B, Trenkwalder C. (2009) Neurochemical Biomarkers In The Differential Diagnosis Of Movement Disorders. https://www.ncbi.nlm.nih.gov/pubmed/19412961
[114] Levin HS, Eisenberg HM, Wigg NR, Kobayashi K. (1982) Memory And Intellectual Ability After Head Injury In Children And Adolescents. https://www.ncbi.nlm.nih.gov/pubmed/7155331
[115] Levin HS, Goldstein FC, High WM Jr, Eisenberg HM. (1988) Disproportionately Severe Memory Deficit In Relation To Normal Intellectual Functioning After Closed Head Injury https://www.ncbi.nlm.nih.gov/pubmed/3225586
[116] Griesbach GS. (2011) Exercise After Traumatic Brain Injury: Is It A Double-Edged Sword? https://www.ncbi.nlm.nih.gov/pubmed/21703583
[117] Archer T. (2012) Influence Of Physical Exercise On Traumatic Brain Injury Deficits: Scaffolding Effect. https://www.ncbi.nlm.nih.gov/pubmed/22183422
[118] Gorman LK, Fu K, Hovda DA, Murray M, Traystman RJ. (1996) Effects Of Traumatic Brain Injury On The Cholinergic System In The Rat. https://www.ncbi.nlm.nih.gov/pubmed/8880609
[119] Sapolsky RM. (2000) Glucocorticoids And Hippocampal Atrophy In Neuropsychiatric Disorders https://www.ncbi.nlm.nih.gov/pubmed/11015810
[120] Dirmaier J, Steinmann M, Krattenmacher T, Watzke B, Barghaan D, Koch U, Schulz H. (2012) Non-Pharmacological Treatment Of Depressive Disorders: A Review Of Evidence-Based Treatment Options. https://www.ncbi.nlm.nih.gov/pubmed/22353197
[121] Byrne A, Byrne DG. (1993) The Effect Of Exercise On Depression, Anxiety And Other Mood States: A Review. https://www.ncbi.nlm.nih.gov/pubmed/8410742
[122] Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, Dimeo FC. (2010) Complex Mental And Physical Activity In Older Women And Cognitive Performance: A 6-Month Randomized Controlled Trial. https://www.ncbi.nlm.nih.gov/pubmed/20418350
[123] Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. (2005) Effects Of Physical Activity On Cognitive Functioning In Middle Age: Evidence From The Whitehall Ii Prospective Cohort Study. https://www.ncbi.nlm.nih.gov/pubmed/16304136
[124] Maillot P, Perrot A, Hartley A. (2012) The Effects Of Video Games On Cognitive Aging https://www.ncbi.nlm.nih.gov/pubmed/22414403
[125] Sentürk UK, Aktekin B, Kuru O, Gündüz F, Demir N, Aktekin MR. (2000) Effect Of Long-Term Swimming Exercise On Somatosensory Evoked Potentials In Rats. https://www.ncbi.nlm.nih.gov/pubmed/11134607
[126] Human neural stem cells from fetal cortex stained for DNA (blue), neuronal (green), and astrocyte (red) markers; James Pires, Brain Cells Inc, USA
[127] Co-culture of primary rat hippocampal neurons and astrocytes stained for βIII-tubulin (green), GFAP (red) and DNA (blue). Janet Anderl, Millipore Corporation, USA
[128] Image depicts neurons of the dentate gyrus, a layer of the hippocampus; slice is from "Brainbow" mice - mice engineered with genes producing fluorescent proteins. Tamily Weissman.
[129] This section is very simplified, and I strongly encourage all interested parties to learn more about the processes and systems of the brain. It is tremendously interesting.
[130] The impact of each individual 'train' can be excitatory or inhibitory to the postsynaptic neuron (or 'station') - I've kept it simple for the sake of the analogy.
[131] LTP is of course only part of the picture. Spike-timing-dependent plasticity (STDP) describes a broader process for the dynamic adjustment of the strength of connections between neurons.
[132] Checking out all the sources ay? NICE!